Special Stains - Thioflavine T
- Amyloid Thioflavine T
- Thioflavine T Technique
- Thioflavine T History
The thioflavine T (TFT) dye combines electrostacially to the carbohydrate fraction bound to the amyloid protein The intensity of the fluorescence allows for good optical visualisation even of minimal deposits with relatively low power objectives. TFT is not specific for amyloid . Prior staining with haematoxylin masks nuclear fluorescence. Other structures such as juxtaglomerular apparatus,fibrinoid, arteriolar hyaline, keratin, paneth cells and zymogen granules also fluoresce with TFT. This effect is minimised by first staining with haematoxylin.
This technique employs the use of a fluorescent microscope for visualisation.
Below is the technique for performing the Thioflavine T stain.
- Take sections to water
- Stain the nuclei in Haematoxylin for 2 minutes.
- Wash in water
- Stain in freshly filtered 1% Thioflavine T for 3 Mins
- Wash in water
- Treat with 1% Acetic Acid for 20 Mins
- Wash in water
- Blot dry
- Mount slides in fluromount.
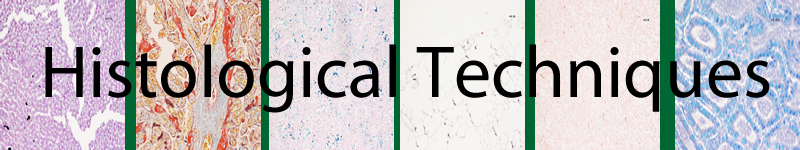
Results
Amyloid and mast cells |
Fluoresce bright yellow/green against an olive background |
Photo - x10 Thioflavine T IF fluorescent section
Thioflavin T is a benzothiazole salt produced by methylation of dehydrothiotoluidine using methanol in the presence of hydrochloric acid. This dye is widely used to visualize and quantify the presence of amyloid, both in vitro and in vivo. When it binds to beta sheet-rich structures, such as those in amyloid aggregates, the dye displays enhanced fluorescence and a characteristic red shift of its emission spectrum. Additional studies have shown fluorescence changes as result of the interaction with double stranded DNA. This change in fluorescent behavior can be caused by many factors that affect the excited state charge distribution of thioflavin T.
Thioflavin T fluorescence is often used as a diagnostic of amyloid structure, but it is not perfectly specific for amyloid. Depending on the particular protein and experimental conditions, thioflavin T may[6] or may not[8][9] undergo a spectroscopic change upon binding to precursor monomers, small oligomers, unaggregated material with a high beta sheet content, or even alpha helix-rich proteins. Conversely, some amyloid fibers do not affect thioflavin T fluorescence,[10] raising the prospect of false negative results.